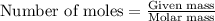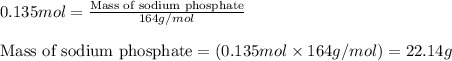Chemistry, 17.02.2020 22:18 kayla114035
Hard water often contains dissolved Ca2 and Mg2 ions. One way to soften water is to add phosphates. The phosphate ion forms insoluble precipitates with calcium and magnesium ions, removing them from solution. A solution is 0.050 M in calcium chloride and 0.085 M in magnesium nitrate. What mass of sodium phosphate would you add to 1.5 L of this solution to completely eliminate the hard water ions.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:02
Which of the following provides support and protection for many insects a) muscle b) skeleton c) spinal cord d) exoskeleton
Answers: 2

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2


Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Hard water often contains dissolved Ca2 and Mg2 ions. One way to soften water is to add phosphates....
Questions

Mathematics, 19.12.2021 23:40





Computers and Technology, 19.12.2021 23:40


English, 19.12.2021 23:40



Social Studies, 19.12.2021 23:50


Health, 19.12.2021 23:50





Social Studies, 19.12.2021 23:50


Computers and Technology, 19.12.2021 23:50

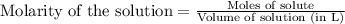 .....(1)
.....(1)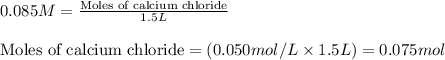
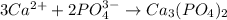
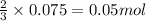 of phosphate ions
of phosphate ions
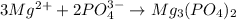
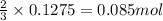 of phosphate ions
of phosphate ions