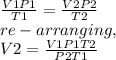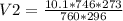Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2


Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
A chemical reaction produced 10.1 cm3 of nitrogen gas at 23 °C and 746 mmHg. What is the volume of t...
Questions




English, 04.12.2019 04:31

English, 04.12.2019 04:31



English, 04.12.2019 04:31




Arts, 04.12.2019 04:31

Mathematics, 04.12.2019 04:31



Biology, 04.12.2019 04:31

History, 04.12.2019 04:31

Mathematics, 04.12.2019 04:31