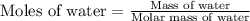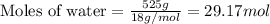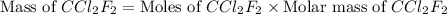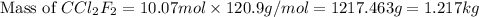Chemistry, 17.02.2020 20:20 therealpr1metime45
A particular refrigerant cools by evaporating liquefied CCl 2F 2. How many kg of the liquid must be evaporated to freeze a tray of water to ice (at zero degrees C)? The tray contains 525 grams water. Molar heat of fusion of ice = 6.01 kJ/mol. Molar heat of vaporization of CCl 2F 2 = 17.4 kJ/mole

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
A particular refrigerant cools by evaporating liquefied CCl 2F 2. How many kg of the liquid must be...
Questions



Biology, 16.10.2020 08:01


Social Studies, 16.10.2020 08:01



Social Studies, 16.10.2020 08:01


History, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01



Mathematics, 16.10.2020 08:01

English, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01


Computers and Technology, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

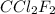 evaporated must be, 1.217 kg
evaporated must be, 1.217 kg