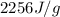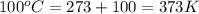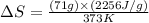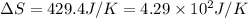Chemistry, 17.02.2020 19:28 kordejah348
What is the entropy change (in J/K) of 71 g of steam at 100°C when it condenses to water at the same temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
What is the entropy change (in J/K) of 71 g of steam at 100°C when it condenses to water at the same...
Questions

Mathematics, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57

History, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57

History, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57

Medicine, 20.06.2020 01:57



Arts, 20.06.2020 01:57


Mathematics, 20.06.2020 01:57

Mathematics, 20.06.2020 01:57




History, 20.06.2020 01:57


Social Studies, 20.06.2020 01:57

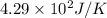
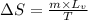
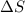 = change in entropy = ?
= change in entropy = ?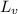 = heat of vaporization of water =
= heat of vaporization of water =