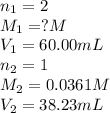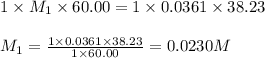Chemistry, 17.02.2020 19:32 Chrisis9987
A 60.00-mL sample of a weak acid is titrated with 0.0361 M NaOH. At the endpoint, it is found that 38.23 mL of titrant was used. What was the concentration of the weak acid?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
A 60.00-mL sample of a weak acid is titrated with 0.0361 M NaOH. At the endpoint, it is found that 3...
Questions

Mathematics, 22.02.2021 05:20

English, 22.02.2021 05:20



Mathematics, 22.02.2021 05:20

Mathematics, 22.02.2021 05:20

Biology, 22.02.2021 05:20


Chemistry, 22.02.2021 05:20

Mathematics, 22.02.2021 05:20

Social Studies, 22.02.2021 05:20

Mathematics, 22.02.2021 05:20


Spanish, 22.02.2021 05:20

History, 22.02.2021 05:20



Biology, 22.02.2021 05:20

English, 22.02.2021 05:20

English, 22.02.2021 05:20

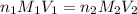
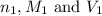 are the n-factor, molarity and volume of acid which is weak acid
are the n-factor, molarity and volume of acid which is weak acid are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.