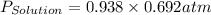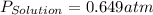Chemistry, 17.02.2020 17:57 Alienhead6187
12. The vapor pressure of water at 90°C is 0.692 atm. What is the vapor pressure (in atm) of a solution made by dissolving 3.68 mole(s) of CsF(s) in 1.00 kg of water? Assume that Raoult's law applies.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

You know the right answer?
12. The vapor pressure of water at 90°C is 0.692 atm. What is the vapor pressure (in atm) of a solut...
Questions

Mathematics, 26.02.2021 20:30


Chemistry, 26.02.2021 20:30

Mathematics, 26.02.2021 20:30

Mathematics, 26.02.2021 20:30

Arts, 26.02.2021 20:30


Mathematics, 26.02.2021 20:30


History, 26.02.2021 20:30

Mathematics, 26.02.2021 20:30



Mathematics, 26.02.2021 20:30



Advanced Placement (AP), 26.02.2021 20:30

Spanish, 26.02.2021 20:30


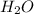 = 1.00 kg = 1000 g
= 1.00 kg = 1000 g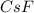 = 3.68 mole
= 3.68 mole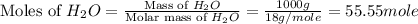
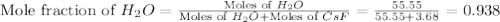
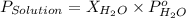
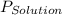 = vapor pressure of solution
= vapor pressure of solution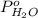 = vapor pressure of water = 0.692 atm
= vapor pressure of water = 0.692 atm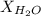 = mole fraction of water = 0.938
= mole fraction of water = 0.938