Chemistry, 17.02.2020 17:27 hiplikedyani
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to form Al2O3(s) at 25°C and 1 atm. ΔHfAl2O3(s) = −1676 kJ/mol HINT: What does ΔHfAl2O3(s) mean?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to fo...
Questions



Health, 16.06.2021 16:30

Mathematics, 16.06.2021 16:30





Mathematics, 16.06.2021 16:30

Mathematics, 16.06.2021 16:30







English, 16.06.2021 16:30



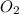 .
.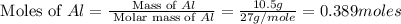
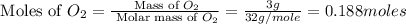
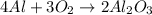
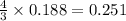 moles of
moles of 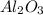
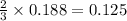 moles of
moles of