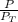A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If the mole fraction of ethanol in the solution is 0.65, calculate its mole fraction in the vapor phase at this temperature. (The vapor pressures of pure ethanol and 1−propanol at 36°C are 108 and 40.0 mmHg, respectively.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1


You know the right answer?
A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If...
Questions










Mathematics, 01.12.2020 01:00


History, 01.12.2020 01:00




English, 01.12.2020 01:00

Chemistry, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Biology, 01.12.2020 01:00

 = 0.65 × 108 mmHg
= 0.65 × 108 mmHg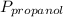 = 0.35× 40.0 mmHg
= 0.35× 40.0 mmHg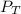 = 70.2 + 4.9 = 75.1 mmHg
= 70.2 + 4.9 = 75.1 mmHg