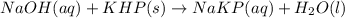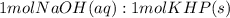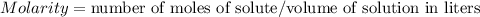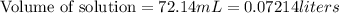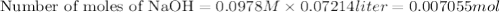Chemistry, 17.02.2020 06:06 asdf334asdf334
Answer the following questions based on the reaction below: NaOH(aq) + KHP(s) --> NaKP(aq)+H2O(I)
A 1.864 g sample of impure KHP was titrated with a 0.0978 M solution of NaOH. To completely react the KHP in the sample, 72.14 mL of base was needed. KHP (potassium hydrogen phthalate, 204.23 g/mol)
a.) How many grams of KHP were in the unknown sample?
b.) What is the percentage of KHP in the unknown sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 11:30
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Answer the following questions based on the reaction below: NaOH(aq) + KHP(s) --> NaKP(aq)+H2O(I)...
Questions


Social Studies, 08.10.2021 03:50


Mathematics, 08.10.2021 03:50

Mathematics, 08.10.2021 03:50






Mathematics, 08.10.2021 03:50

Mathematics, 08.10.2021 03:50

Physics, 08.10.2021 03:50

Business, 08.10.2021 03:50


Biology, 08.10.2021 04:00

History, 08.10.2021 04:00


Biology, 08.10.2021 04:00

English, 08.10.2021 04:00