Chemistry, 16.02.2020 19:41 queenkimm26
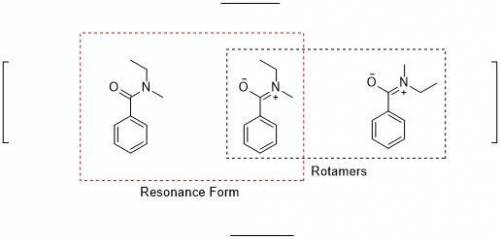
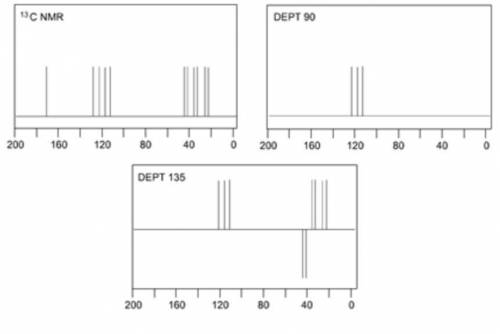
When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm–1. The 13C NMR and DEPT spectra are provided below. Draw the structure of the product as the resonance contributor lacking any formal charges.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1


You know the right answer?
When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/...
Questions

English, 23.09.2019 05:30


History, 23.09.2019 05:30




Mathematics, 23.09.2019 05:30


Spanish, 23.09.2019 05:30

History, 23.09.2019 05:30

Mathematics, 23.09.2019 05:30


Spanish, 23.09.2019 05:30

Mathematics, 23.09.2019 05:30


Biology, 23.09.2019 05:30

History, 23.09.2019 05:30