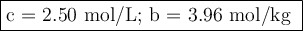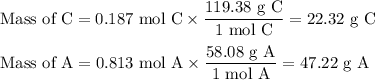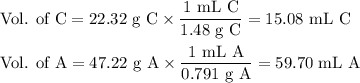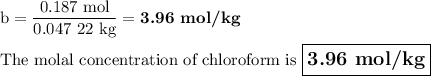Chemistry, 16.02.2020 02:36 keegandudley
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fraction of chloroform is 0.187. The densities of chloroform and acetone are 1.48 g/mL and 0.791 g/mL, respectively.
Calculate the molarity and molality of the solution.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2


Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

You know the right answer?
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fracti...
Questions


Chemistry, 08.01.2021 18:40




Mathematics, 08.01.2021 18:40

Mathematics, 08.01.2021 18:40


Mathematics, 08.01.2021 18:40



English, 08.01.2021 18:40





Geography, 08.01.2021 18:40


Mathematics, 08.01.2021 18:40