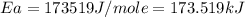For the gas phase isomerization of methyl cis-cinnamate, cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHC OOCH3 the rate constant at 637 K is 1.88×10-4 s-1 and the rate constant at 679 K is 1.43×10-3 s-1. The activation energy for the gas phase isomerization of methyl cis-cinnamate is

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
For the gas phase isomerization of methyl cis-cinnamate, cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHC OOCH3 t...
Questions

Mathematics, 08.09.2021 03:10

Mathematics, 08.09.2021 03:10


Mathematics, 08.09.2021 03:20


Mathematics, 08.09.2021 03:20


Mathematics, 08.09.2021 03:20


English, 08.09.2021 03:20







Mathematics, 08.09.2021 03:20

Social Studies, 08.09.2021 03:20

Mathematics, 08.09.2021 03:20

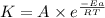
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0512/2904/6d953.png)
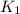 = rate constant at
= rate constant at 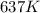 =
= 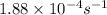
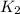 = rate constant at
= rate constant at  =
= 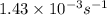
 = activation energy for the reaction = ?
= activation energy for the reaction = ? = initial temperature =
= initial temperature = 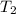 = final temperature =
= final temperature = ![\log (\frac{1.43\times 10^{-3}}{1.88\times 10^{-4}})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{637}-\frac{1}{679}]](/tpl/images/0512/2904/23370.png)
![0.88=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{637}-\frac{1}{679}]](/tpl/images/0512/2904/11d95.png)