Chemistry, 15.02.2020 02:59 Nyasiahenry
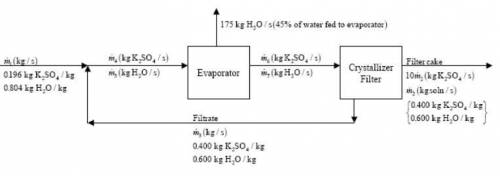
An evaporation–crystallization process of the type described in Example 4.5-2 is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 19.6 wt% K2SO4. The wet filter cake consists of solid K2SO4 crystals and a 40.0 wt% K2SO4 solution, in a ratio 10 kg crystals/kg solution. The filtrate, also a 40.0% solution, is recycled to join the fresh feed. Of the water fed to the evaporator, 45.0% is evaporated. The evaporator has a maximum capacity of 175 kg water evaporated/s. Calculate the maximum production rate of solid K2SO4, the rate at which fresh feed must be supplied to achieve this production rate.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


You know the right answer?
An evaporation–crystallization process of the type described in Example 4.5-2 is used to obtain soli...
Questions


Mathematics, 29.05.2020 03:57

English, 29.05.2020 03:57

History, 29.05.2020 03:57

Mathematics, 29.05.2020 03:57

Biology, 29.05.2020 03:57



Social Studies, 29.05.2020 03:57


History, 29.05.2020 03:57

History, 29.05.2020 03:57



Mathematics, 29.05.2020 03:57

Mathematics, 29.05.2020 03:57

Engineering, 29.05.2020 03:57

Mathematics, 29.05.2020 03:57

Mathematics, 29.05.2020 03:57

Advanced Placement (AP), 29.05.2020 03:57

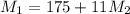
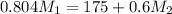
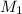 = 220.77 kg/s
= 220.77 kg/s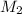 = 4.16 kg/s
= 4.16 kg/s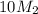 = 10*4.16 = 416 kg/s
= 10*4.16 = 416 kg/s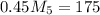
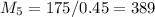 kg/s
kg/s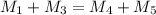
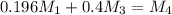
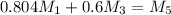
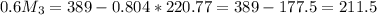
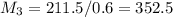 kg/s
kg/s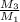 = 352.5/220.77 = 1.6
= 352.5/220.77 = 1.6