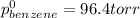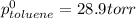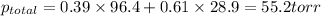Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Benzene has a formula of C6H6 and a vapor pressure of 96.4 torr at 298 K. Toluene has a formula of C...
Questions

Mathematics, 26.03.2020 04:30

Biology, 26.03.2020 04:30




Mathematics, 26.03.2020 04:30




English, 26.03.2020 04:30



Mathematics, 26.03.2020 04:31

Mathematics, 26.03.2020 04:31




Mathematics, 26.03.2020 04:31


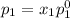 and
and 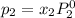
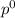 = pressure in the pure state
= pressure in the pure state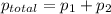
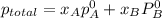
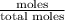
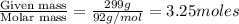
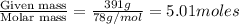
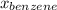 = mole fraction of benzene =
= mole fraction of benzene =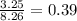
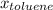 =mole fraction of toluene = (1-0.39) = 0.61
=mole fraction of toluene = (1-0.39) = 0.61