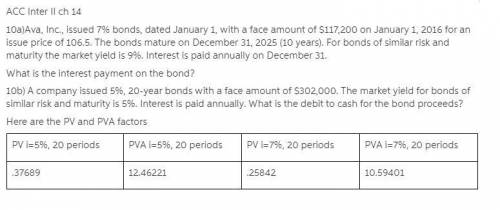
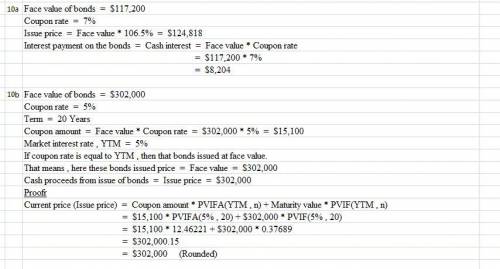
Ava, Inc., issued 7% bonds, dated January 1, with a face amount of $117,200 on January 1, 2016 for an issue price of 106.5. The bonds mature on December 31, 2025 (10 years). For bonds of similar risk and maturity the market yield is 9%. Interest is paid annually on December 31.
What is the interest payment on the bond?
10b) A company issued 5%, 20-year bonds with a face amount of $302,000. The market yield for bonds of similar risk and maturity is 5%. Interest is paid annually. What is the debit to cash for the bond proceeds?
Here are the PV and PVA factors
PV i=5%, 20 periods
PVA i=5%, 20 periods
PV i=7%, 20 periods
PVA i=7%, 20 periods
.37689
12.46221
.25842
10.59401

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Ava, Inc., issued 7% bonds, dated January 1, with a face amount of $117,200 on January 1, 2016 for a...
Questions

Mathematics, 17.04.2020 01:53




Mathematics, 17.04.2020 01:53




Mathematics, 17.04.2020 01:53

Engineering, 17.04.2020 01:54

Mathematics, 17.04.2020 01:54





Mathematics, 17.04.2020 01:54


Mathematics, 17.04.2020 01:54

Mathematics, 17.04.2020 01:54

Chemistry, 17.04.2020 01:54