
A solution of HNO 3 is standardized by reaction with pure sodium carbonate. 2 H + + Na 2 CO 3 ⟶ 2 Na + + H 2 O + CO 2 A volume of 26.66 ± 0.06 mL of HNO 3 solution was required for complete reaction with 0.9479 ± 0.0007 g of Na 2 CO 3 , (FM 105.988 ± 0.001 g/mol ). Find the molarity of the HNO 3 solution and its absolute uncertainty. Note: Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
A solution of HNO 3 is standardized by reaction with pure sodium carbonate. 2 H + + Na 2 CO 3 ⟶ 2 Na...
Questions

Biology, 13.04.2020 09:00

History, 13.04.2020 09:01

English, 13.04.2020 09:01

Mathematics, 13.04.2020 09:01

Biology, 13.04.2020 09:02

Business, 13.04.2020 09:02




Mathematics, 13.04.2020 09:19





Biology, 13.04.2020 09:19


English, 13.04.2020 09:19

Mathematics, 13.04.2020 09:20


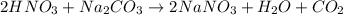
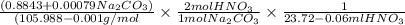
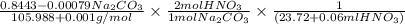
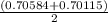 = 0.703495
= 0.703495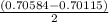
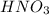 .
.

