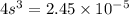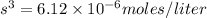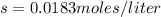Chemistry, 14.02.2020 21:21 romerok568
The K sp for barium fluoride, BaF2, is 2.45 × 10-5. What is the molar solubility of barium fluoride?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
The K sp for barium fluoride, BaF2, is 2.45 × 10-5. What is the molar solubility of barium fluoride?...
Questions

English, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

English, 27.04.2021 16:40


Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

Arts, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40





Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40



Health, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

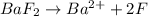
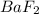 gives 2 moles of
gives 2 moles of 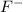 and 1 mole of
and 1 mole of 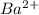
![K_sp=[Ba^{2+}][F^{-}]^2](/tpl/images/0511/8336/a05b2.png)
![2.45\times 10^{-5}=[s][2s]^2](/tpl/images/0511/8336/a4b76.png)