
Chemistry, 14.02.2020 18:25 Chynadoll94
The product of a Lewis acid-base reaction is a(n) , a single species that contains a new covalent bond. The Lewis acid-base definition focuses on the donation or acceptance of an electron pair to form a new covalent bond in an adduct, the product of an acid-base reaction. Lewis bases the electron pair, and Lewis acids it.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

You know the right answer?
The product of a Lewis acid-base reaction is a(n) , a single species that contains a new covalent bo...
Questions












Chemistry, 08.11.2019 23:31


English, 08.11.2019 23:31

English, 08.11.2019 23:31



Mathematics, 08.11.2019 23:31


Biology, 08.11.2019 23:31

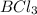 (B has vacant 2p orbital for electron acceptance)
(B has vacant 2p orbital for electron acceptance)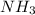 (N has energetically available lone pair)
(N has energetically available lone pair)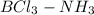 ) where a new covalent bond is formed between B and N.
) where a new covalent bond is formed between B and N.

