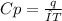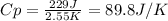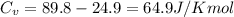Chemistry, 14.02.2020 17:25 postorivofarms
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temperature of the sample increases by 2.55 K. Assuming that under these conditions nitrogen behaves as an ideal gas, what is the value of the molar heat capacity at constant volume of N2(g)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temp...
Questions

Mathematics, 20.04.2021 05:20

Mathematics, 20.04.2021 05:20

Mathematics, 20.04.2021 05:20



Mathematics, 20.04.2021 05:20

Mathematics, 20.04.2021 05:20


Mathematics, 20.04.2021 05:20

Advanced Placement (AP), 20.04.2021 05:20

Chemistry, 20.04.2021 05:20

Mathematics, 20.04.2021 05:20


English, 20.04.2021 05:20


Mathematics, 20.04.2021 05:20

Mathematics, 20.04.2021 05:20

History, 20.04.2021 05:20

History, 20.04.2021 05:20

 is 64.9 J/Kmol
is 64.9 J/Kmol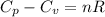
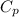 = heat capacity at constant pressure
= heat capacity at constant pressure 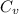 = heat capacity at constant volume
= heat capacity at constant volume