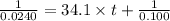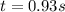Chemistry, 14.02.2020 06:11 cchavcchav2944
At a certain temperature the rate of this reaction is second order in with a rate constant of Suppose a vessel contains at a concentration of . Calculate how long it takes for the concentration of to decrease to . You may assume no other reaction is important. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1

Chemistry, 23.06.2019 12:30
Question 1 (true/false worth 4 points) (03.06 lc) an induced dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized. true false
Answers: 2

Chemistry, 23.06.2019 15:00
What is the volume in liters of 7500 g of helium atoms. assume stp conditions.
Answers: 1
You know the right answer?
At a certain temperature the rate of this reaction is second order in with a rate constant of Suppos...
Questions






Computers and Technology, 05.12.2020 01:00



Mathematics, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00


Social Studies, 05.12.2020 01:00


Health, 05.12.2020 01:00


History, 05.12.2020 01:00

History, 05.12.2020 01:00


Mathematics, 05.12.2020 01:00

Social Studies, 05.12.2020 01:00

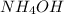 with a rate constant of
with a rate constant of 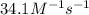 .
. 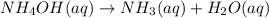
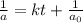
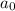 = initial concentration = 0.100 M
= initial concentration = 0.100 M