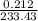Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
A solution contains an unknown mass of dissolved barium ions. When sodium sulfate is added to the so...
Questions

Mathematics, 09.12.2021 09:30



History, 09.12.2021 09:30

Mathematics, 09.12.2021 09:30


Mathematics, 09.12.2021 09:30



Mathematics, 09.12.2021 09:30


History, 09.12.2021 09:30



English, 09.12.2021 09:30





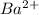 and
and 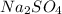 are added then white precipitate forms. And, reaction equation for this is as follows.
are added then white precipitate forms. And, reaction equation for this is as follows.
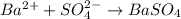
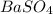 is 233.43.
is 233.43.