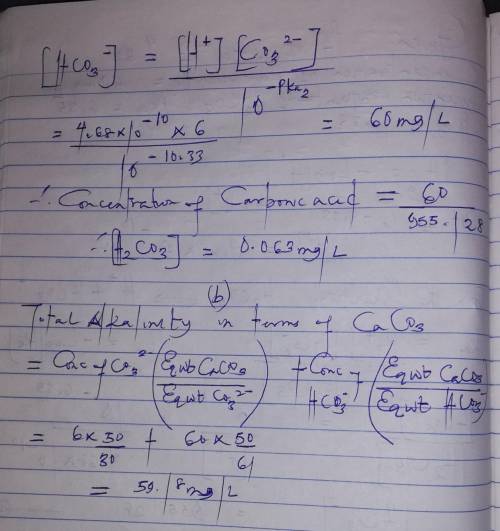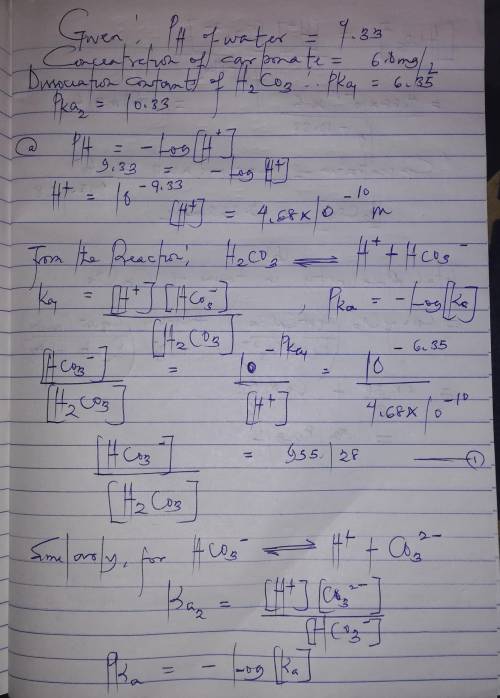The pH of a water sample is measured to be 9.33. The concentration of carbonate (i. e., CO32-) is measured to be 6.0 mg/L. Assume the system is closed to the atmosphere and the temperature is 25 oC. Given that pKa1 and pKa2 of H2CO3 are 6.35 and 10.33, respectively,
a. Calculate the concentrations of bicarbonate and carbonic acid, respectively. Express your answers both in mol/L and mg/L.
b. Calculate the alkalinity of the water both in meq/L and mg/L as CaCO3.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
The pH of a water sample is measured to be 9.33. The concentration of carbonate (i. e., CO32-) is me...
Questions

Geography, 04.01.2021 01:00

Mathematics, 04.01.2021 01:00





History, 04.01.2021 01:00


Mathematics, 04.01.2021 01:00

Mathematics, 04.01.2021 01:00

Mathematics, 04.01.2021 01:00

Biology, 04.01.2021 01:00



Physics, 04.01.2021 01:00

Social Studies, 04.01.2021 01:00


Social Studies, 04.01.2021 01:00