
Chemistry, 14.02.2020 02:44 SKSKSKSKGKUFHjk
Write the equilibrium-constant expression for the reactionA(s)+3B(l)↽−−⇀2C(aq)+D(aq)A (s)+3B(l)↽−−⇀2C(aq)+D(aq)in terms of [A], [B], [C], and [D] as needed. Note that KcKc, which is sometimes symbolized as KeqKeq, denotes that the equilibrium constant is expressed using molar concentrations. For this question, KcKc means the same thing as KeqKeq. Kc=.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
Write the equilibrium-constant expression for the reactionA(s)+3B(l)↽−−⇀2C(aq)+D(aq)A (s)+3B(l)↽−−⇀2...
Questions

Computers and Technology, 15.07.2019 11:00


Mathematics, 15.07.2019 11:00




Mathematics, 15.07.2019 11:00


Mathematics, 15.07.2019 11:00

Mathematics, 15.07.2019 11:00






Mathematics, 15.07.2019 11:00




English, 15.07.2019 11:00

![K_{c} = [\text{C}]^{2}[\text{[D]}](/tpl/images/0511/0111/07ffc.png)
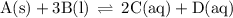
![K_{c} = \dfrac{[\text{Products}]}{[\text{Reactants}]}](/tpl/images/0511/0111/68d06.png)
![K_{c} = [\textbf{C}]^{\mathbf{2}}\textbf{[D]}](/tpl/images/0511/0111/3e70c.png)


