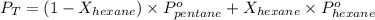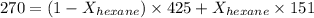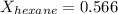Chemistry, 14.02.2020 00:58 evelynrrojas461
A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 270 torr . Pure pentane and hexane have vapor pressures of 425 torr and 151 torr, respectively, at room temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pr...
Questions



Computers and Technology, 04.12.2020 17:20

Physics, 04.12.2020 17:20


History, 04.12.2020 17:20


Mathematics, 04.12.2020 17:20



English, 04.12.2020 17:20


Social Studies, 04.12.2020 17:20





History, 04.12.2020 17:20


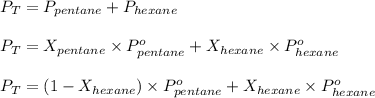
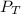 = total vapor pressure = 270 torr
= total vapor pressure = 270 torr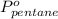 = vapor pressure of pure pentane = 425 torr
= vapor pressure of pure pentane = 425 torr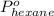 = vapor pressure of pure hexane= 151 torr
= vapor pressure of pure hexane= 151 torr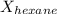 = mole fraction of hexane = ?
= mole fraction of hexane = ?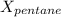 = mole fraction of pentane
= mole fraction of pentane