
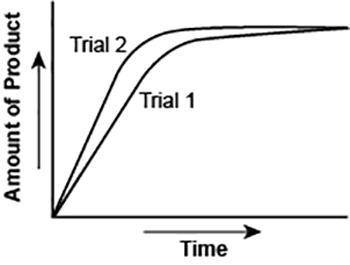
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a *lower* concentration of the reactant? (5 points)
Trial 1, because the average rate of the reaction is lower.
Trial 1, because this reaction lasted for a longer duration than Trial 2.
Trial 2, because this reaction was initially fast and later slowed down.
Trial 2, because the volume of product formed per unit time was higher.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2

Chemistry, 23.06.2019 13:00
What mass of ca(oh)2 is needed to make 1250ml of a .75m solution?
Answers: 3

You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions

Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20

Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20

Social Studies, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20




Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20

Mathematics, 21.01.2021 16:20



