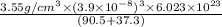Chemistry, 13.02.2020 22:42 cyrusshakeri4814
A hypothetical AX type of ceramic material is known to have a density of 3.55 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.39 nm. The atomic weights of the A and X elements are 90.5 and 37.3 g/mol, respectively. On the basis of this information, which one of the following crystal structures is possible for this material?a) fluoriteb) zinc blendc) cesium chloride

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
A hypothetical AX type of ceramic material is known to have a density of 3.55 g/cm3 and a unit cell...
Questions





Mathematics, 15.08.2020 01:01


English, 15.08.2020 01:01


Mathematics, 15.08.2020 01:01

Chemistry, 15.08.2020 01:01


Mathematics, 15.08.2020 01:01



Computers and Technology, 15.08.2020 01:01


Mathematics, 15.08.2020 01:01



Mathematics, 15.08.2020 01:01


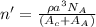
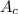 = molecular weight of cation = 90.5 g/mol
= molecular weight of cation = 90.5 g/mol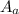 = molecular weight of anion = 37.3 g/mol
= molecular weight of anion = 37.3 g/mol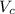 = volume of cubic cell = 3.55
= volume of cubic cell = 3.55 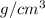
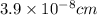
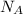 = Avogadro's number =
= Avogadro's number = 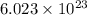
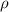 = density = 3.55
= density = 3.55