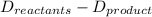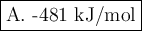Chemistry, 13.02.2020 20:53 camcollins00
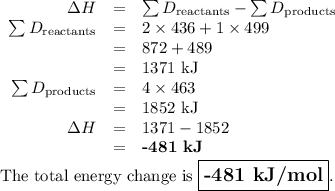
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:
H-H bond: 436 kJ/mol
O-O double bond: 499 kJ/mol
H-O bond: 463 kJ/mol
A. -481 kJ/mol
B. + 445 kJ/mol
C. +63 kJ/mol
D. -730.5 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
What is the total energy change for the following reaction: 2H2 + O2 -> 2H2O?
Given:...
Given:...
Questions


Physics, 01.08.2019 16:00

Biology, 01.08.2019 16:00

Physics, 01.08.2019 16:00



English, 01.08.2019 16:00




History, 01.08.2019 16:00









 = ( A ) ; - 481 kJ/mol
= ( A ) ; - 481 kJ/mol