
Chemistry, 13.02.2020 19:57 CrusaderLord
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibrium in favor of products. b. Addition of SCN-(aq) will shift the equilibrium in favor of reactants. c. Addition of Cl-(aq) will shift the equilibrium in favor of reactants.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
For the reaction Fe3+(aq) + SCN-(aq) Fe(SCN)2+(aq). a. Addition of SCN-(aq) will shift the equilibri...
Questions

Physics, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

English, 08.03.2021 14:00



Mathematics, 08.03.2021 14:00


Health, 08.03.2021 14:00

English, 08.03.2021 14:00


History, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00


Advanced Placement (AP), 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Advanced Placement (AP), 08.03.2021 14:00

Mathematics, 08.03.2021 14:00


English, 08.03.2021 14:00

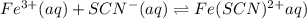
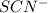 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

