Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
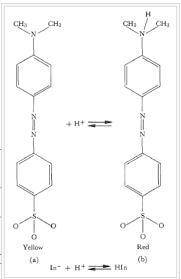
Indicators change color depending on whether they are in a protonated (HIn) or unprotonated (In-) fo...
Questions


Physics, 03.12.2021 15:30


Mathematics, 03.12.2021 15:30

Biology, 03.12.2021 15:30

Biology, 03.12.2021 15:30

Business, 03.12.2021 15:30

Mathematics, 03.12.2021 15:30

English, 03.12.2021 15:30

Chemistry, 03.12.2021 15:30

Mathematics, 03.12.2021 15:40

English, 03.12.2021 15:40

Mathematics, 03.12.2021 15:40


Mathematics, 03.12.2021 15:40

Mathematics, 03.12.2021 15:40


Biology, 03.12.2021 15:40