1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW

Chemistry, 13.02.2020 18:36 samantha9430
The proposed mechanism for a reaction is
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
2. I-(aq) + HClO(aq) => HIO(aq) + Cl-(aq) FAST
3. OH-(aq) + HIO(aq) => H2O(l) + IO-(aq) FAST
Which of the following would be a rate law for the reaction?
1. rate = k[ClO-][H2O][I-][OH-]
2. rate = k[ClO-][H2O]
3. rate = k[OH-][HIO]
4. rate = k[I-][HClO]
5. rate = k[ClO-][H2O][I-]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

You know the right answer?
The proposed mechanism for a reaction is
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW
Questions



Biology, 25.06.2019 12:00

Mathematics, 25.06.2019 12:00




Biology, 25.06.2019 12:00






History, 25.06.2019 12:00

English, 25.06.2019 12:00





Mathematics, 25.06.2019 12:00

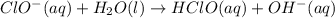 is a slow step reaction.
is a slow step reaction.![k[ClO^{-}][H_{2}O]](/tpl/images/0510/0437/87324.png)


