Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
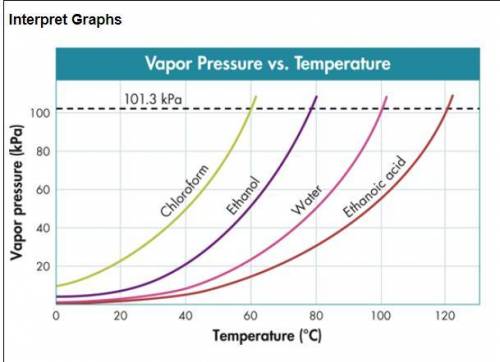
Use the figure below to determine to boiling point of -Chloroform at 80 kPa -Ethanol at 20 kPa -Etha...
Questions

Mathematics, 25.07.2020 04:01


English, 25.07.2020 04:01

Mathematics, 25.07.2020 04:01




Mathematics, 25.07.2020 04:01


Arts, 25.07.2020 04:01





Mathematics, 25.07.2020 04:01

Mathematics, 25.07.2020 04:01