
Chemistry, 13.02.2020 06:14 saintsfan2004
Calculate the kinetic energy in j/mol of particles with mass 32.5 g/mol with an average speed of 491 m/sec.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Calculate the kinetic energy in j/mol of particles with mass 32.5 g/mol with an average speed of 491...
Questions

Mathematics, 05.01.2021 04:50

Chemistry, 05.01.2021 04:50

Mathematics, 05.01.2021 04:50


English, 05.01.2021 04:50

English, 05.01.2021 04:50





Mathematics, 05.01.2021 04:50



English, 05.01.2021 04:50

Mathematics, 05.01.2021 04:50

History, 05.01.2021 04:50

Mathematics, 05.01.2021 04:50


Mathematics, 05.01.2021 04:50

Health, 05.01.2021 04:50

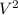
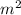 /
/
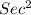 / mol
/ mol

