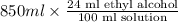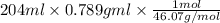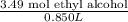Chemistry, 13.02.2020 05:54 haileyparrill703
A 850.0 mL bottle of Listerine is of a 24 % (v/v) ethyl alcohol. If the density of ethyl alcohol is 0.789 g/mL and the molar mass is 46.07 g/mol, calculate the molarity of ethyl alcohol in Listerine.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
A 850.0 mL bottle of Listerine is of a 24 % (v/v) ethyl alcohol. If the density of ethyl alcohol is...
Questions



English, 03.12.2019 08:31

Mathematics, 03.12.2019 08:31


Mathematics, 03.12.2019 08:31

History, 03.12.2019 08:31




Social Studies, 03.12.2019 08:31



Biology, 03.12.2019 08:31

Mathematics, 03.12.2019 08:31

Chemistry, 03.12.2019 08:31

Mathematics, 03.12.2019 08:31



Mathematics, 03.12.2019 08:31