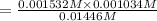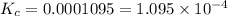Chemistry, 13.02.2020 03:48 emmaguentherp3hjd3
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this: (aq)(l)(aq)(aq) At a certain temperature, a chemist finds that a reaction vessel containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition: compound amount Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this: (aq)(l)(aq...
Questions

Chemistry, 06.05.2020 04:41

Mathematics, 06.05.2020 04:41




Social Studies, 06.05.2020 04:41





Social Studies, 06.05.2020 04:41



Computers and Technology, 06.05.2020 04:41



Geography, 06.05.2020 04:41



 = 1.62 g
= 1.62 g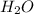 = 516 g
= 516 g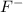 = 0.163 g
= 0.163 g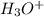 = 0.110 g
= 0.110 g .
.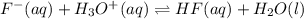
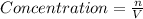
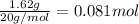
![[HF]=\frac{0.081 mol}{5.6 L}=0.01446 M](/tpl/images/0509/5531/82215.png)
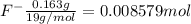
![[F^-]=\frac{0.008579 mol}{5.6 L}=0.001532 M](/tpl/images/0509/5531/21915.png)
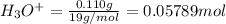
![[H_3O^+]=\frac{0.05789 mol}{5.6 L}=0.001034 M](/tpl/images/0509/5531/e5735.png)
![K_c=\frac{[F^-][H_3O^+]}{[HF]}](/tpl/images/0509/5531/d3418.png)