Chemistry, 13.02.2020 02:23 bonetanne7171
The density at 20 ∘C of a 0.828 M solution of acetic acid in water is 1.0052 g/mL. The molar mass of acetic acid, CH3CO2H, is 60.05 g/mol. What is the molality of the solution?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
The density at 20 ∘C of a 0.828 M solution of acetic acid in water is 1.0052 g/mL. The molar mass of...
Questions




Mathematics, 14.09.2021 17:00

Mathematics, 14.09.2021 17:00


Mathematics, 14.09.2021 17:00

Mathematics, 14.09.2021 17:00

Mathematics, 14.09.2021 17:00


Chemistry, 14.09.2021 17:00

Mathematics, 14.09.2021 17:00



Social Studies, 14.09.2021 17:00



Mathematics, 14.09.2021 17:00

Biology, 14.09.2021 17:00

![d=M[\frac{1}{m}+\frac{M_b}{1000}]](/tpl/images/0509/4030/a25dd.png)
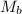 = molar mass of solute (acetic acid) = 60.05 g/mole
= molar mass of solute (acetic acid) = 60.05 g/mole![1.0052g/ml=0.828mol/L\times [\frac{1}{m}+\frac{60.05g/mole}{1000}]](/tpl/images/0509/4030/d7754.png)