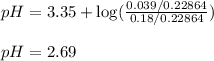Problem PageQuestion An analytical chemist is titrating of a solution of nitrous acid with a solution of . The of nitrous acid is . Calculate the pH of the acid solution after the chemist has added of the solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to decimal places.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
Problem PageQuestion An analytical chemist is titrating of a solution of nitrous acid with a solutio...
Questions





Mathematics, 15.04.2020 01:19



Mathematics, 15.04.2020 01:19












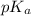 of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.
of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.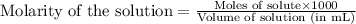
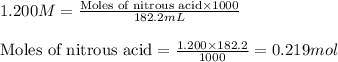


![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0509/3412/e4eea.png)
![pH=pK_a+\log(\frac{[KNO_2]}{[HNO_2]})](/tpl/images/0509/3412/fa3ae.png)
![[KNO_2]=\frac{0.039}{0.22864}](/tpl/images/0509/3412/7262b.png)
![[HNO_2]=\frac{0.18}{0.22864}](/tpl/images/0509/3412/eee2b.png)