Chemistry, 13.02.2020 00:34 halbrookc7082
A solution is prepared by mixing 0.10 L of 0.12 M sodium chloride with 0.23 L of a 0.18 M magnesium chloride solution. What volume of a silver nitrate solution (at 0.200 M) is required to precipitate all the Cl– ion in the solution as AgCl (s)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
A solution is prepared by mixing 0.10 L of 0.12 M sodium chloride with 0.23 L of a 0.18 M magnesium...
Questions



Mathematics, 08.01.2021 01:00



Mathematics, 08.01.2021 01:00

Mathematics, 08.01.2021 01:00


English, 08.01.2021 01:00

Business, 08.01.2021 01:00

Mathematics, 08.01.2021 01:00

Arts, 08.01.2021 01:00

Mathematics, 08.01.2021 01:00


Mathematics, 08.01.2021 01:00



Mathematics, 08.01.2021 01:00


English, 08.01.2021 01:00

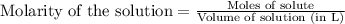
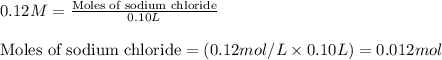
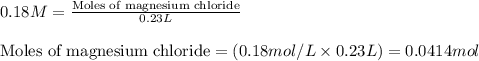
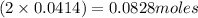
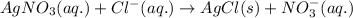
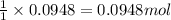 of silver nitrate
of silver nitrate