
Chemistry, 12.02.2020 23:28 purplepig12
If the freezing point of the solution had been incorrectly read 0.3 °C lower than the true freezing point, would the calculated molar mass of the solute have been too high or too low? Explain.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
If the freezing point of the solution had been incorrectly read 0.3 °C lower than the true freezing...
Questions

Mathematics, 26.10.2021 17:50




Biology, 26.10.2021 17:50

Mathematics, 26.10.2021 17:50

Mathematics, 26.10.2021 17:50

History, 26.10.2021 17:50





Mathematics, 26.10.2021 17:50


English, 26.10.2021 17:50



History, 26.10.2021 17:50

Mathematics, 26.10.2021 17:50

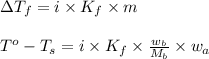
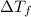 = change in freezing point
= change in freezing point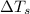 = freezing point of solution
= freezing point of solution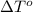 = freezing point of water
= freezing point of water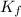 = freezing point constant
= freezing point constant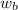 = mass of solute
= mass of solute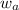 = mass of solvent
= mass of solvent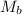 = molar mass of solute
= molar mass of solute

