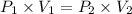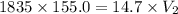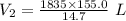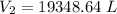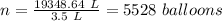Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
A 155.0 −L helium tank contains pure helium at a pressure of 1835 psi and a temperature of 298 K. Ho...
Questions







Mathematics, 20.08.2019 08:10

History, 20.08.2019 08:10

History, 20.08.2019 08:10

Biology, 20.08.2019 08:10

Mathematics, 20.08.2019 08:10



Biology, 20.08.2019 08:10


Mathematics, 20.08.2019 08:10

History, 20.08.2019 08:10

Mathematics, 20.08.2019 08:10

Mathematics, 20.08.2019 08:10