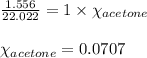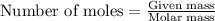Chemistry, 12.02.2020 05:58 mjlchance367
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor pressure of water over the solution is lowered by 1.556 kPa. Given the vapor pressure of water at 65°C is 25.022 kPa, what is the mass of acetone added?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor press...
Questions


Mathematics, 22.09.2021 16:00



Mathematics, 22.09.2021 16:00

Mathematics, 22.09.2021 16:00

Computers and Technology, 22.09.2021 16:00

Social Studies, 22.09.2021 16:00

Arts, 22.09.2021 16:00

Mathematics, 22.09.2021 16:00

Biology, 22.09.2021 16:00



Geography, 22.09.2021 16:00

Mathematics, 22.09.2021 16:00





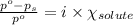
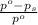 = relative lowering in vapor pressure = 1.556 kPa
= relative lowering in vapor pressure = 1.556 kPa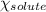 = mole fraction of solute = ?
= mole fraction of solute = ?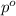 = vapor pressure of pure water = 22.022 kPa
= vapor pressure of pure water = 22.022 kPa