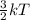A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort the conditions based on the gas described. Some may have more than one answer, but noe will have the same answer.
1) Nitrogen has the greater value
2) Xenon has the greater value
3) Both gases have the same value
A. Gas that exerts the greater partial pressure.
B. Gas for which the molecules or atoms have the greatest average velocity
C. Gas that would have a higher rate of effusion through a small hole opened in the flask.
D. Gas for which the molecules or atoms have the greatest average of kinetic energy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon. Sort th...
Questions

History, 19.05.2020 16:19

Mathematics, 19.05.2020 16:19

Mathematics, 19.05.2020 16:19


Computers and Technology, 19.05.2020 16:19




Mathematics, 19.05.2020 16:19


English, 19.05.2020 16:19

Mathematics, 19.05.2020 16:19



Biology, 19.05.2020 16:19

History, 19.05.2020 16:19

History, 19.05.2020 16:19

History, 19.05.2020 16:19

Mathematics, 19.05.2020 16:19