

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
At a concentration of 1 M, the weak acid HNO 2 is 2% ionized, and the pH of the solution is 1.7. Wha...
Questions

Social Studies, 21.02.2022 14:00

Social Studies, 21.02.2022 14:00

Mathematics, 21.02.2022 14:00



Mathematics, 21.02.2022 14:00

English, 21.02.2022 14:00


Mathematics, 21.02.2022 14:00

English, 21.02.2022 14:00


History, 21.02.2022 14:00

Arts, 21.02.2022 14:00


Mathematics, 21.02.2022 14:00





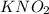 dissolved into the solution then the equilibrium will shift in the left direction.
dissolved into the solution then the equilibrium will shift in the left direction.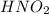 is:
is: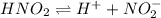
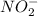 then the equilibrium will shift in the left direction.
then the equilibrium will shift in the left direction.

