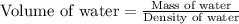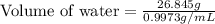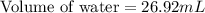Chemistry, 12.02.2020 04:41 carlosgc19
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the total mass to be 32.634g. He then filled the flask with water, weighed again, and obtained a mass of 59.479g. At the temperature of the water, he found that its density was 0.9973 g/mL. a.) What was the mass of the water? (show work)b.) What was the volume of the water? (Show work)c.) What was the volume of the flask? (show work)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 08:10
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1
You know the right answer?
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the t...
Questions


English, 20.03.2021 06:00

Social Studies, 20.03.2021 06:00

Mathematics, 20.03.2021 06:00


Mathematics, 20.03.2021 06:00







Chemistry, 20.03.2021 06:00


Mathematics, 20.03.2021 06:00




Social Studies, 20.03.2021 06:00

Arts, 20.03.2021 06:00