
Chemistry, 12.02.2020 04:39 winterblanco
1. Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.) A. BrF3 B. H3O C. PCl3 D. SO42- E. SF4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
1. Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar....
Questions




Mathematics, 07.03.2021 02:00

Mathematics, 07.03.2021 02:00



History, 07.03.2021 02:00


Mathematics, 07.03.2021 02:00

English, 07.03.2021 02:00


Mathematics, 07.03.2021 02:00



Business, 07.03.2021 02:00

History, 07.03.2021 02:00

Mathematics, 07.03.2021 02:00

Mathematics, 07.03.2021 02:00

Mathematics, 07.03.2021 02:00

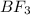
![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0508/1323/9e987.png)
![\text{Number of electrons}=\frac{1}{2}\times [3+3]=3](/tpl/images/0508/1323/ce6da.png)
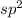 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.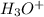
![\text{Number of electrons}=\frac{1}{2}\times [6+3-1]=4](/tpl/images/0508/1323/9514c.png)
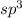 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.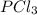
![\text{Number of electrons}=\frac{1}{2}\times [5+3]=4](/tpl/images/0508/1323/5f4d9.png)
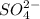
![\text{Number of electrons}=\frac{1}{2}\times [6+2]=4](/tpl/images/0508/1323/2c46b.png)
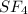
![\text{Number of electrons}=\frac{1}{2}\times [6+4]=5](/tpl/images/0508/1323/f1dca.png)
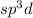 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.

