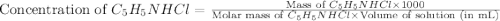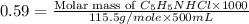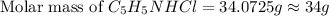Chemistry, 12.02.2020 03:34 leneenmarshall3125
A chemistry graduate student is given of a dimethylamine solution. Dimethylamine is a weak base with . What mass of should the student dissolve in the solution to turn it into a buffer with pH ? You may assume that the volume of the solution doesn't change when the is dissolved in it. Be sure your answer has a unit symbol, and round it to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
A chemistry graduate student is given of a dimethylamine solution. Dimethylamine is a weak base with...
Questions

Advanced Placement (AP), 01.02.2021 20:00

Mathematics, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00





Mathematics, 01.02.2021 20:00

History, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00


Social Studies, 01.02.2021 20:00

Biology, 01.02.2021 20:00


Biology, 01.02.2021 20:00

Mathematics, 01.02.2021 20:00

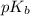 .
.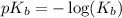
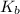 in this expression, we get:
in this expression, we get: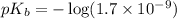
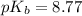
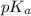
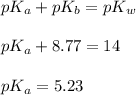
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0508/0206/e961a.png)
![pH=pK_a+\log \frac{[C_5H_5N]}{[C_5H_5NHCl]}](/tpl/images/0508/0206/7f262.png)
![4.76=5.23+\log (\frac{0.20}{[C_5H_5NHCl]})](/tpl/images/0508/0206/63ae1.png)
![[C_5H_5NHCl]=0.59M](/tpl/images/0508/0206/438d4.png)