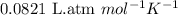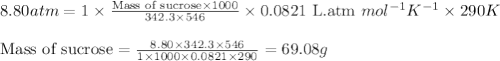Chemistry, 12.02.2020 03:24 cuppykittyy
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an osmotic pressure of 8.80 atm at 290 K ? (Assume the density of the solution to be equal to the density of the solvent.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an o...
Questions

Geography, 07.07.2019 17:00


History, 07.07.2019 17:00

History, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00


History, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00



Geography, 07.07.2019 17:00


Physics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00


Mathematics, 07.07.2019 17:00

Chemistry, 07.07.2019 17:00

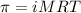
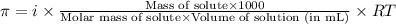
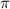 = osmotic pressure of the solution = 8.80 atm
= osmotic pressure of the solution = 8.80 atm