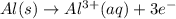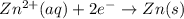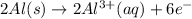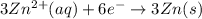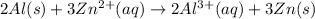The half-reactions for the oxidation-reduction reaction between Al(s) and Zn₂ (aq) are represented above. Based on the half-reactions, what is the coefficient for Al(s) if the equation for the oxidation-reduction reaction is balanced with the smallest whole-number coefficients?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
The half-reactions for the oxidation-reduction reaction between Al(s) and Zn₂ (aq) are represented a...
Questions


English, 24.02.2021 22:20

Mathematics, 24.02.2021 22:20

Mathematics, 24.02.2021 22:20

Mathematics, 24.02.2021 22:20



English, 24.02.2021 22:20

History, 24.02.2021 22:20


Spanish, 24.02.2021 22:20

Mathematics, 24.02.2021 22:20

Mathematics, 24.02.2021 22:20



Advanced Placement (AP), 24.02.2021 22:20



Mathematics, 24.02.2021 22:20