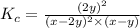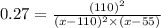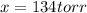Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOC...

Chemistry, 12.02.2020 02:45 ghaithalhamdani
Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOCl(g)
Kp=0.27 at 700 K
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl was measured to be 110 torr. What were the initial partial pressures of NO and Cl2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1


Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
You know the right answer?
Questions

Physics, 06.03.2022 15:50

Computers and Technology, 06.03.2022 15:50

Biology, 06.03.2022 15:50




Chemistry, 06.03.2022 15:50


English, 06.03.2022 15:50

Mathematics, 06.03.2022 15:50


Chemistry, 06.03.2022 16:00


Mathematics, 06.03.2022 16:00

Mathematics, 06.03.2022 16:00

Mathematics, 06.03.2022 16:00

Social Studies, 06.03.2022 16:00



Mathematics, 06.03.2022 16:00

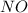 and
and 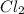 are 134 torr each.
are 134 torr each.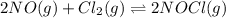
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0507/8534/56950.png)