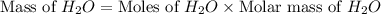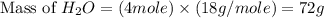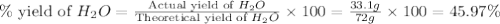Chemistry, 26.08.2019 12:50 dbenitezmontoya3
Two moles of oxygen gas is reacted with hydrogen gas to produce water in this reaction: 2 h2 + o2 → 2 h2o in the lab you actually made 33.1 grams of water. what is the percent yield? 218.10% 91.80% 45.87% 54.50%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1
You know the right answer?
Two moles of oxygen gas is reacted with hydrogen gas to produce water in this reaction: 2 h2 + o2 →...
Questions

Mathematics, 09.03.2020 07:26


Biology, 09.03.2020 07:27


Chemistry, 09.03.2020 07:27



Mathematics, 09.03.2020 07:27

Biology, 09.03.2020 07:27


Mathematics, 09.03.2020 07:28

Mathematics, 09.03.2020 07:28

Mathematics, 09.03.2020 07:28

Mathematics, 09.03.2020 07:29



Mathematics, 09.03.2020 07:29

Mathematics, 09.03.2020 07:29

Mathematics, 09.03.2020 07:30

History, 09.03.2020 07:30

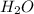 is, 45.97 %
is, 45.97 %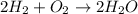
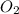 react to give 2 moles of
react to give 2 moles of 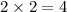 moles of
moles of