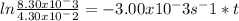The conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C CH3NC(g)CH3CN(g) is first order in CH3NC with a rate constant of 3.00×10-3 s-1. If the initial concentration of CH3NC is 4.30×10-2 M, the concentration of CH3NC will be 8.30×10-3 M after s have passed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
The conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C CH3NC(g)CH3CN(g) is f...
Questions



Biology, 13.11.2020 19:10





Mathematics, 13.11.2020 19:10

Computers and Technology, 13.11.2020 19:10

Spanish, 13.11.2020 19:10


Biology, 13.11.2020 19:10



Mathematics, 13.11.2020 19:10


Health, 13.11.2020 19:10

Mathematics, 13.11.2020 19:10


Mathematics, 13.11.2020 19:10

![ln \frac{[A]_t}{[A]_0} = -kt](/tpl/images/0507/3637/bde68.png)
![[A]_t](/tpl/images/0507/3637/82372.png) is the concentration of the reactant in the time t,
is the concentration of the reactant in the time t, ![[A]_0](/tpl/images/0507/3637/7075c.png) is the initial concentration of the reactant, k is rate constant and t is time. Replacing with values of the problem:
is the initial concentration of the reactant, k is rate constant and t is time. Replacing with values of the problem: